Funded by the Ministry of Higher Education, Research and Innovation (MoHERI), a team led by Dr. Haytham Ali of Sultan Qaboos University (SQU) has developed a ready to use, rapid, and sensitive colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) based assay for the diagnosis of Covid-19 infection.

The assay can be utilized at its current status as a screening assay with the advantages of being simpler, quicker (20-30 minutes), and cheaper than the qRT-PCR.
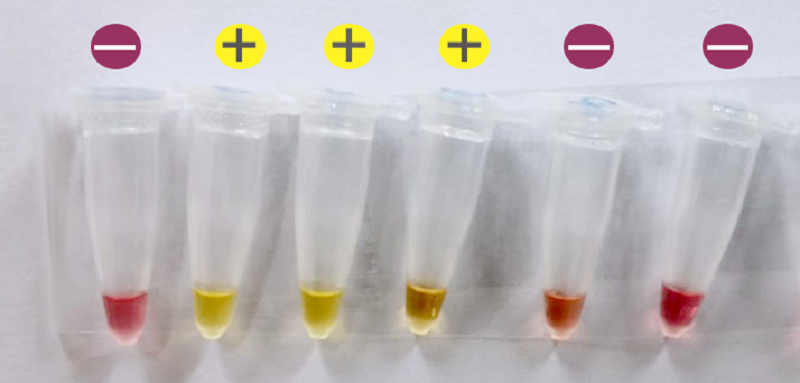
The research idea is derived from the fact that a rapid and sensitive Covid-19 test is in need of the prevention and virus. “The colorimetric assay is highly sensitive and was able to detect as low as 80 viral genome copies with possible improvement through further adjustments.” Dr. Haytham Ali said.
Three primer sets were designed by Dr. Timothy Holton from SQU (co-principal investigator) based on the complete genome sequences of forty-one SARS-CoV-2 isolates from Oman, targeting the Spike protein gene and the M gene.
The primer set (CoV_S23258) was found to be the most sensitive and specific among the three designed sets. The RT-LAMP assay was validated by testing 145 COVID-19 clinical samples obtained from SQU Hospital with a sensitivity of 96.9 percent and specificity of 94.7 percent when compared to the validated Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) assay.
The RT-LAMP assay specificity was tested against SARS-CoV Frankfurt-1 RNA virus and avian coronaviruses as they tested negative with the developed assay.
The research team examined the possibility of directly using saliva samples spiked with SARS-CoV-2 RNA that gave preliminary excellent results with the developed assay.
Dr. Haytham’s team is trying to convert the test into a lyophilized (freeze-dried) ready-to-use assay, where water and template need to be added to run the test. Results can be obtained by reading the color change from red to yellow. “Once we validate the lyophilized assay, we will proceed of implementing the assay as a point of care test in clinics, airports, and other places,” Dr. Haytham added.
Oman Observer is now on the WhatsApp channel. Click here