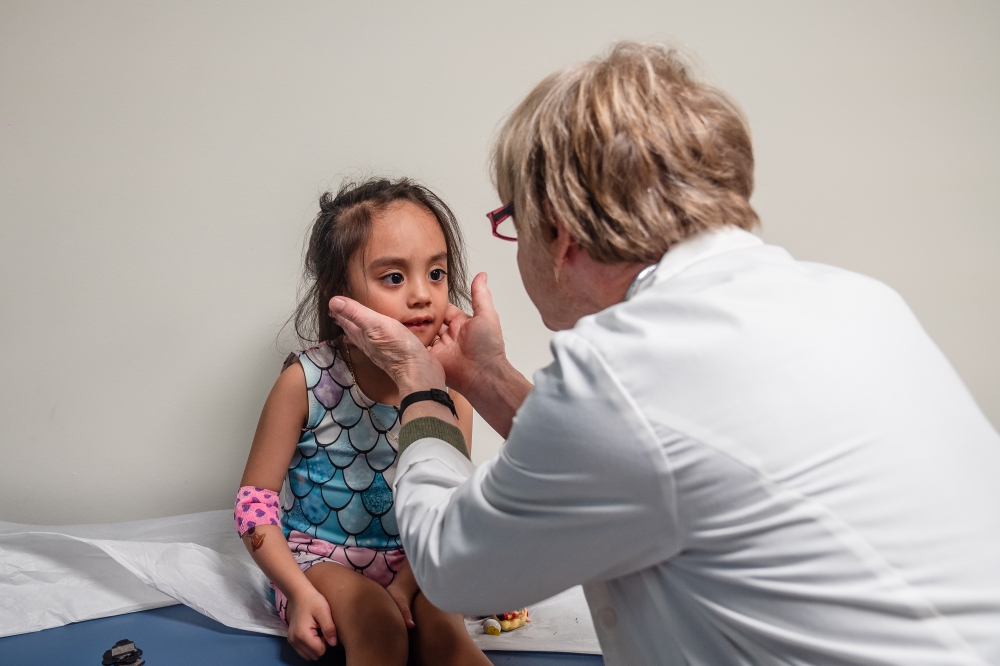It looked like a scene from the TV crime show “CSI.” Dr. Jane C. Burns was peering into a multiheaded microscope at the San Diego County medical examiner’s office, scrutinizing autopsy samples from an array of mysterious deaths.
This one was from the heart of a 20-year-old jujitsu fighter who was last seen at the gym and was found dead in his bed two days later. There were no signs of foul play or self-harm.
The blood vessel tissue on the slide looked abnormal. Burns turned to the examiner: “I think this was likely one of mine.”
Burns is an expert in a rare childhood illness called Kawasaki disease, which is the most common cause of acquired heart disease in children worldwide. It is also one of pediatric medicine’s greatest mysteries: No one knows what causes it.
And Burns, who leads the investigations at the Kawasaki Disease Research Center at the University of California, San Diego, has devoted her life to solving that mystery.
The condition, which usually occurs in children under 5, is easy to miss: There is no diagnostic test, and its symptoms — a high fever, rash, red cracked lips and a “strawberry tongue” — look to many doctors like scarlet fever, measles or a tick-borne illness, despite its signature distinction of bloodshot eyes.
Kawasaki disease can often resolve itself in a matter of weeks. But without the right treatment, about a quarter of patients develop aneurysms of the coronary arteries, which can lead to sudden heart attacks and death, even years or decades later — as happened to the young man whose tissue Burns was analyzing.
Burns and other scientists believe that children inherit some level of susceptibility to it from their parents and that the condition is then brought on by something they breathe in, whether a virus, a bacteria or a toxin. Climate scientists wonder if global warming could also be broadening the disease’s scope.
Her freezers hold the world’s largest biobank of Kawasaki disease samples, and she scours for hints — in the living and the dead — in hopes of eventually discovering the culprit. She believes that by determining the cause, researchers could develop diagnostic tests that would lead to more timely treatment and prevent more deaths.
To get there, she has built a most unlikely network of detectives — an oceanographer, a statistician, a cardiologist, a historian, a forensic pathologist, a microbiologist, an anthropologist, and others — each with specific expertise. And after 40 years of sleuthing, she believes, the team might just have the tools to complete the mission.
“This is a quest indeed — a puzzle — but Jane is doggedly persistent,” said Daniel Cayan, a climatologist at the Scripps Institution of Oceanography who joined Burns’ team to help investigate how climate variability could be influencing the disease. “Here you have a marvelous organizer and fearless ringleader, pulling on every string and angle, chasing this.”
Answers could not come at a better time. The rate of Kawasaki disease in Japan, where it is most rampant, is increasing at an alarming rate, and doctors in the United States are now seeing a jump in cases after years when the rate remained steady, most likely because some children were protected from exposure during pandemic-era social distancing measures.
This year, doctors at Rady Children’s Hospital-San Diego, which admits the largest number of Kawasaki disease patients in the United States, have seen double the usual number of cases. Hospitals in Boston, Colorado, and Chicago have also reported surges.
“Every day in our country, somewhere, a child with KD is being misdiagnosed,” Burns said. “Before now, we didn’t have the tools and the teams and the samples and the data to attack this disease. Now we do, so let’s get moving.”
She added, “It’s taken a half-century to get here, but now we’re ready to roll.”
From a Children’s Playroom to the Atmosphere
Kawasaki disease first captured Burns’ attention in 1981 when she was a senior medical resident and cared for a feverish 3-month-old baby with mysterious rashes. The baby died. Burns watched the autopsy and, when the chest was opened, could not believe her eyes: Aneurysms beaded on the surface of the infant’s heart.
The baby’s parents went door to door collecting donations in loose bills and eventually handed $1,500 to Burns in a brown bag, asking her to research the disease. Burns persuaded so many senior professors to help her that, for their first meeting, they crammed into the hospital ward’s play space, squatting in children’s chairs to brainstorm a plan: The head of neurology would volunteer a fellow to run electroencephalograms; an immunologist would study the role of T-cells. Burns’ career as master collaborator was born.
In the early years of her investigation, the epidemiology of Kawasaki disease looked much like a classic infection passed between people. There were three large nationwide epidemics in Japan in the late 1970s and the 1980s, each so drastic that it suggested a novel agent was moving through a highly susceptible population. Each was followed by a plateau period, typically of several years.
But in the 1990s, things began to look strange. The number of school-age children with the disease in Japan kept climbing, despite a falling birthrate, hinting that more and more children were being exposed to its mystery cause each year.
Burns looked into an array of potential triggers, including carpet cleaning. She and her husband, Dr. John Gordon, a cardiologist treating dozens of adult patients with missed cases of Kawasaki disease, hosted the physician who had first identified the disease, Dr. Tomisaku Kawasaki, at their San Diego home. Burns went to Japan to interview every living person who had ever been involved in recognizing a new disease there. But there was little funding to pursue such work.
By 2000, a student of Burns’ noticed that Kawasaki disease cases in San Diego climbed whenever it rained. Burns partnered with Cayan, the climatologist, and Japanese researchers to discover that cases in Japan rose and fell with seasonal rhythms and that, in contrast to what is seen with person-to-person outbreaks, the case levels were always oddly consistent across broad swaths of the country.
Later, she and colleagues including a European climate scientist, Xavier Rodó, analyzed records of more than 247,000 patients in Japan to discover that the biggest outbreaks of the disease had something peculiar in common: They had all occurred when large-scale wind currents were blowing in from Central Asia. When those winds reached Hawaii and California, cases climbed there, too.
It was then that Burns and her colleagues began to argue that whatever was causing Kawasaki disease in children was not just passing from person to person; it was possibly being blown across the world on the wind.
It seemed unlikely to some that living particles could survive a journey through the icy troposphere — but Burns, who routinely froze viruses to preserve them in the lab, couldn’t shake the theory.
The team waited until the agent was expected to be present in the air and then dispatched an airplane to fly over Japan and collect air samples. With the help of Ian Lipkin, a microbiologist at Columbia, they processed the filters and found a type of fungus called Candida. But it was still just an association — not a sure cause — and funding once again ran dry.
A Natural Experiment
In recent years, Burns has recruited new members to her polymathic team, including Dr. Jennifer Burney, an environmental scientist at UC San Diego, and Laurel DeHaan, a data analyst, who came aboard to help them analyze the records of almost half a million Kawasaki disease patients in Japan over three decades, stratifying them by age and region.
What they found was bizarre: The rate of cases among children over 3 years old had increased fivefold in recent decades, but the rate among infants had remained relatively flat the whole time. What’s more, the seasonal cycle of cases for the age groups was completely different.
The annual peak for toddlers shifted in the mid-2010s — around the time the Japanese government dramatically expanded daycare options for families.
In 2020 came a natural experiment. The dawn of the COVID pandemic brought school closures, and Kawasaki disease among children in the United States fell by 28%. But most common respiratory viruses that are transmitted between children faded away almost entirely, and the disease did not — in fact, the number of children under 12 months with the disease did not change much at all, hinting that some exposure inside homes continued to affect infants.
The atmosphere can be “complicated and chaotic,” said Charles Copeland, the newest recruit, who uses historical records and supercomputer weather models to estimate the globe’s wind patterns for every hour of the day dating back to the 1970s. (He reverse-tracks particles using a program called HYSPLIT, based on software developed during the Cold War to track radioactive particles back to their origins.) The goal, he said in one of the recent team meetings, is to figure out whether seemingly random bursts of Kawasaki disease in history were related to particularly anomalous wind patterns.
“To really try to untangle this and get to the heart of whether this is a wind story, we really have to ask the right questions,” he said. “If you ask the wrong questions — under the wrong assumptions — I think you will get the wrong answer.”
A Missed Case, or Two
At the Kawasaki Disease Clinic at Rady Children’s Hospital-San Diego, led by Burns, caring for children affected by Kawasaki disease is always linked to the search for the cause.
On a recent Wednesday morning, Dr. Kirsten Dummer, a pediatric cardiologist, was examining the heart scans of a 2-year-old who showed signs of a large aneurysm on the right side of the heart.
“The biggest question from parents is: How did this happen? How did my child get this? In every patient room, that’s what they fundamentally want to know,” she said. “Year after year after year, they come back and ask us, ‘Do you guys know more yet?’”
Burns, who has continued to see patients herself, said those inquiries motivated her.
“If we were all Ph.D.s in the laboratory working on the etiology of Kawasaki disease,” there would be a different pace to it, Burns said. “But there’s an urgency to it, because we’re going back and forth, from the lab to the patients, saying, ‘Damn it, I need to answer this question.’ It matters, because it matters to these people.”
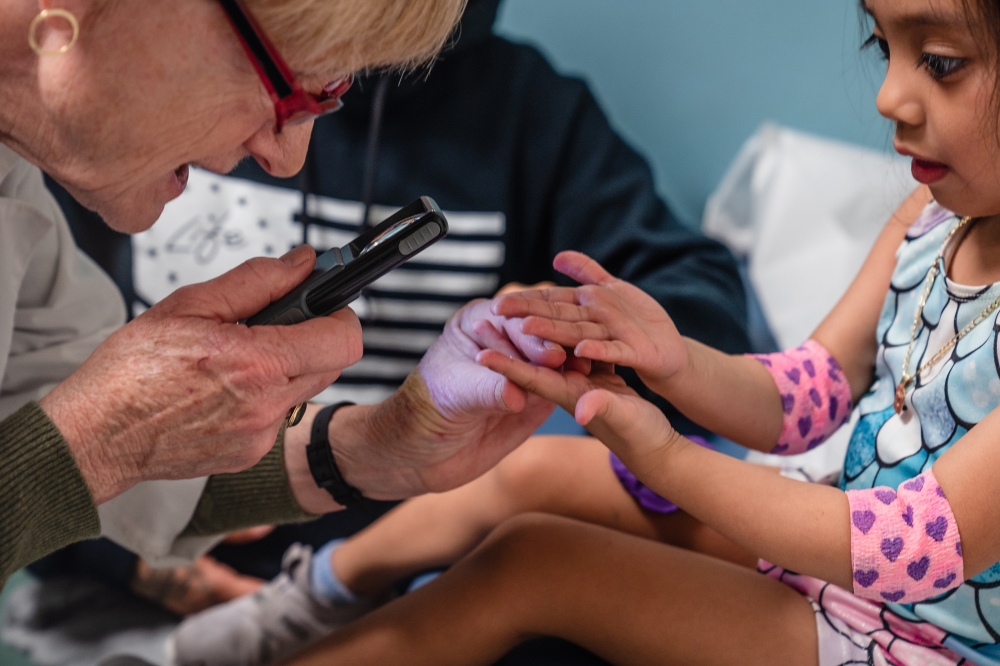
Later that morning, Inez Maldonado Diega, a 4-year-old in a mermaid outfit, rolled out balls of Play-Doh with her mother as Burns broke the news. Seventeen days ago, the girl’s pediatrician’s office had missed her case of Kawasaki disease. An echocardiogram had come back clear — a sign that her heart was so far healthy — but she still had a fever, which meant the disease could be lingering.
“I wish we had seen her sooner,” Burns said, listening to Inez’s heartbeat. She requested genetic samples for her biobank from both Inez and her mother, explaining that children are believed to inherit a susceptibility to the disease from their parents.
Inez’s mother, Tiara Diega, assured Burns that she had never had Kawasaki disease as a child — just scarlet fever. Burns raised her eyebrows and asked Diega to phone her mother on speakerphone.
Had Diega had bloodshot eyes during her infection all those years ago, she asked Diega’s mother? Yes, the mother said. Burns exhaled slowly.
“That wasn’t scarlet fever,” she said.
For a moment, the room was quiet — Diega still holding a patty of Play-Doh in midair — as the risks to both mother and daughter sunk in. Then Burns referred Diega for a cardiac scan of her own — to see whether a grave danger had been brewing all these years.
This article originally appeared in The New York Times.
Oman Observer is now on the WhatsApp channel. Click here