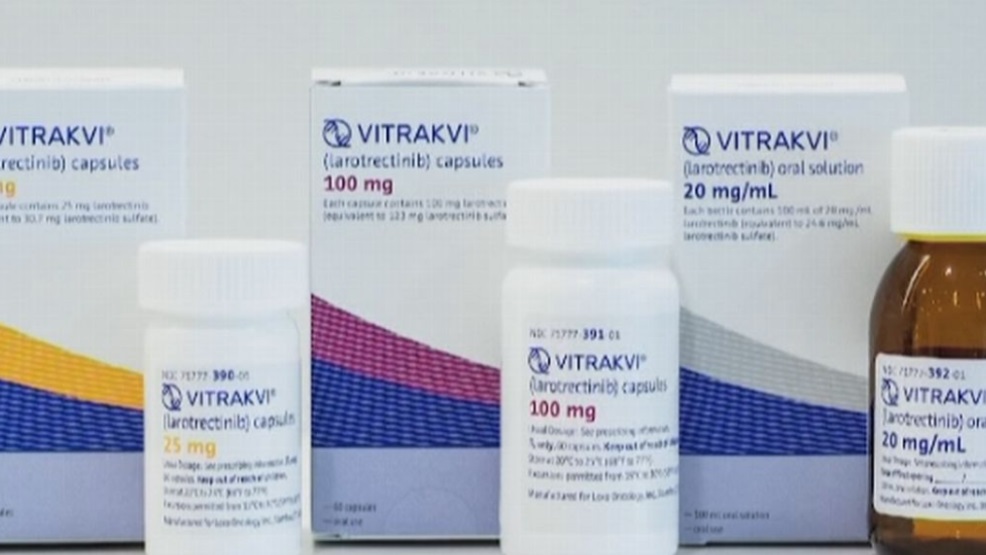
Muscat: The Ministry of Health (MOH) in Oman has issued a clarification on the US Food and Drug Administration's approval for the Larotrectinab drug, known as Vitrakvi
In a statement, the Ministry of Health said the drug was tested on a limited number of patients by the US FDA (55 patients, including 12 patients under the age of 18).
Preliminary results showed that 73 per cent of these patients responded to the treatment within the first nine months of treatment , but the percentage started to decline gradually until it reached 39 per cent during the first year.
The ministry said the drug is works in an iterative manner through preventing the growth of tumors caused by a rare genetic disorder (genetic mutation causing some kinds of cancers) assocaited with this genetic defect but not all the cancer types.
MOH said the drug has a number of side effects that have been observed who have been treated by this medicine, fatigue, nausea, vomiting, high liver enzymes, dizziness, diarrhea and constipation.
Oman Observer is now on the WhatsApp channel. Click here